Silent blood disorders, serious consequences: What patients must know
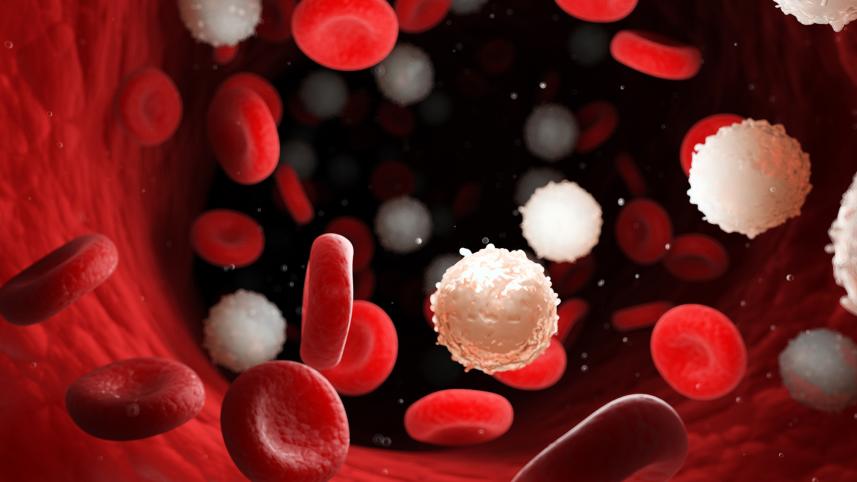
Clin Prof Ng Heng Joo, Head and Senior Consultant in Haematology at Singapore General Hospital (SGH), recently visited Dhaka, Bangladesh, to conduct academic sessions and engage with local clinicians. In this interview with The Daily Star, he discusses common blood disorders in South Asia, advances in haematology, life-threatening clotting conditions, and the growing role of diagnostics and technology in improving patient outcomes.

Thalassaemia remains a major public health concern in South Asia. What key messages would you like to share with patients and families?
Thalassaemia is a genetic blood disorder that remains highly prevalent in South Asia, including Bangladesh. One of the most important priorities is public awareness—understanding how thalassaemia is inherited, what it means to be a carrier, and why early diagnosis matters.
Carrier detection is especially important for young adults planning families. If both partners carry the same type of thalassaemia gene, there is a risk of having children with severe forms of the disease. In such situations, couples may choose to undergo prenatal testing and counselling to make informed decisions.
For patients with severe thalassaemia, early diagnosis allows timely blood transfusions to reduce complications of chronic anaemia. As healthcare systems develop, improved access to iron chelation therapy is equally critical, as repeated transfusions can cause harmful iron overload. Ultimately, awareness today protects the health of future generations.
Some countries have successfully reduced severe thalassaemia through national screening programmes. Why has this been challenging in South Asia?
Successful prevention requires leadership from healthcare professionals and public health authorities. Screening programmes need to be embedded at key life stages—particularly during pregnancy or pre-marital counselling.
In Singapore, for example, pregnant women undergo routine blood tests that can indicate thalassaemia. If a woman is identified as a carrier, her spouse is tested. When both parents carry the same gene, counselling and further testing can be offered.
Such programmes depend on policy support, trained personnel, and public trust. Where these elements are fragmented or under-resourced, screening becomes inconsistent. Awareness must begin with healthcare providers, who play a vital role in diagnosis, counselling, and referral.
Bone marrow transplantation is often discussed as a curative option. Who are the ideal candidates?
Bone marrow—or stem cell—transplantation is primarily indicated for severe beta-thalassaemia (thalassaemia major), particularly in patients who are transfusion-dependent.
Several factors determine suitability. Ideally, transplantation should be performed in childhood or early adolescence, usually below the age of 14, before iron overload causes organ damage. Patients must also be well-chelated prior to transplant.
Donor availability is another major factor. The best outcomes are seen when a matched sibling donor is available. In selected cases, alternative donors—including parents—may be considered, although this carries higher risks, such as graft-versus-host disease. When successful, transplantation can cure up to 90 per cent of patients.
What is the long-term outlook for patients after a successful transplant?
If a transplant is successful and complications such as graft-versus-host disease do not occur, patients can gradually stop immunosuppressive medications within one to two years. Once off these drugs, their infection risk decreases significantly.
In such cases, life expectancy can be comparable to that of healthy individuals. Essentially, the patient may be considered cured of thalassaemia.
You manage complex blood cancers such as leukaemia and myeloma. How have recent advances improved outcomes?
Advances in diagnostics have transformed haematology. We can now classify blood cancers more accurately and, importantly, predict how patients are likely to respond to treatment—a process known as prognostication.
This allows for tailored therapy. Patients with favourable prognostic markers may receive less intensive treatment, reducing side effects, while those at higher risk can be treated more aggressively. Targeted therapies have further expanded our options.
Another major advance is the ability to detect very low levels of residual disease after treatment. If no disease is detected, therapy may be safely stopped. If minimal disease persists, treatment can be intensified early to reduce the risk of relapse.
Deep vein thrombosis and pulmonary embolism can be fatal. What warning signs should people be aware of?
These conditions are collectively known as venous thromboembolism. Most cases begin as deep vein thrombosis, usually in the legs. Warning signs include pain and swelling in one leg, particularly in the calf.
Risk factors include prolonged immobility, recent surgery or injury, cancer, hormonal therapy, and inherited clotting disorders. If a clot travels to the lungs, it can cause sudden chest pain, breathlessness, or collapse, which is a medical emergency.
Recognising risk factors and early symptoms is critical for prompt treatment and prevention of serious complications.
Does diabetes increase the severity of these clotting conditions?
Diabetes alone does not significantly increase the risk of deep vein thrombosis. However, if diabetes is associated with other conditions—such as immobility, obesity, or cardiovascular disease—the overall risk may increase. Most patients, even with diabetes-related nerve problems, can still detect leg swelling or discomfort.
Haemophilia and bleeding disorders often go undiagnosed in low-resource settings. What practical steps can improve care?
Two key areas need attention. First is awareness among healthcare providers, including doctors, nurses, and community health workers, particularly in rural areas. Unexplained bleeding, especially into joints or muscles, should prompt referral.
Second is access to basic diagnostic tests, such as PT and APTT, which measure blood clotting time and can be performed in many laboratories. Abnormal results should trigger referral to specialised centres for confirmatory testing.
Establishing referral networks between rural facilities and specialist centres can significantly improve early diagnosis and outcomes.
Many patients travel abroad for diagnostic confirmation. How important is laboratory accuracy in haematology?
Accurate diagnosis is fundamental. While routine blood tests are generally reliable in many countries, highly specialised tests—such as those for certain blood cancers—require advanced expertise and technologies.
Training laboratory specialists, encouraging international collaboration, and developing platforms for second opinions can strengthen diagnostic confidence. If diagnostic accuracy improves locally, many patients can receive appropriate care without travelling abroad.
Artificial intelligence is gaining attention in diagnostics. How do you see its role in haematology?
AI and machine learning hold promise, particularly in pattern recognition, such as identifying abnormal cells under the microscope. Some systems can already detect and count leukaemia cells with high accuracy.
However, AI is only as good as the data it receives. Proper sample collection, preparation, and processing remain essential. AI will complement—not replace—skilled laboratory professionals.



 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel.
Comments