Single-dose drug shows promise against drug-resistant gonorrhoea
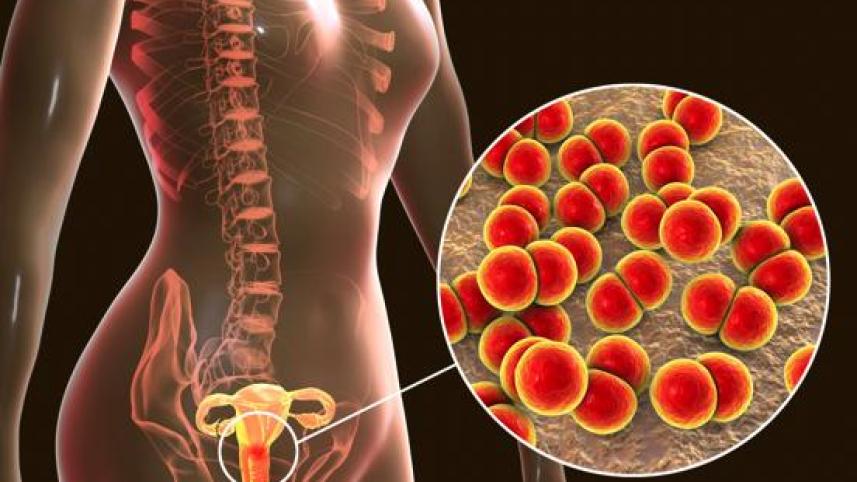
A new oral medicine called zoliflodacin is showing strong potential as a treatment for antibiotic-resistant gonorrhoea, according to a phase 3 clinical trial published in The Lancet. The study found that a single dose of the drug worked just as well as the current standard treatment, which involves an injection of ceftriaxone followed by oral azithromycin.
Gonorrhoea is one of the most common sexually transmitted infections in the world, affecting more than 82 million people every year. Treating it is becoming increasingly difficult because the bacteria that cause the infection are developing resistance to existing antibiotics. This makes new treatment options urgently needed. Zoliflodacin could help slow the spread of drug-resistant gonorrhoea and make treatment easier and more accessible, especially in settings where injections are difficult to provide.
The international trial involved over 900 participants from five countries: the United States, South Africa, Thailand, Belgium and the Netherlands. Participants were given either a single dose of zoliflodacin or the standard two-drug treatment. Results showed that more than 90% of genital gonorrhoea infections were cured in those who received the new medication.
The drug was well tolerated, with side effects similar to those seen with current treatments, such as mild stomach discomfort. Importantly, no serious safety concerns were reported during the trial.
Zoliflodacin is now awaiting approval from the U.S. Food and Drug Administration (FDA). If approved, experts say it could significantly strengthen global efforts to control antibiotic-resistant gonorrhoea, support community-based care, and help protect the sexual and reproductive health of millions of people worldwide.


 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel.
Comments